Twenty years ago, two Rice University chemists won the Nobel Prize for a revolutionary idea about carbon molecules.
The discovery of Buckyballs, a new form of carbon that ushered in the era of nanotechnology and won a Nobel Prize, happened largely by accident.
In 1985, Rice University chemists Robert Curl and Richard Smalley hosted British chemist Harry Kroto for a series of experiments in Houston. Kroto had a theory about how long carbon chains were formed in the atmospheres of carbon-rich giant stars, and Smalley had built a laser beam apparatus that could vaporize molecules and test the theory.
Over 10 days, the three professors and three graduate students conducted tests in which they vaporized carbon molecules with Smalley’s laser beam apparatus and then measured how the carbon atoms clustered together. To their surprise, in addition to the long chain molecules they were seeking, they found a high number of clusters consisting of 60 carbon atoms.
The professors tasked their graduate students with finding ways of changing the parameters of the experiment to increase the number of C60 molecules and tried to theorize what their structure would look like. They knew the structure had to be something more stable, like a sphere, that would protect the bonds between the carbon atoms from being easily broken.
“What was the chemical structure?” Curl recalled in his Rice office earlier this month. “How can you put 60 carbon atoms together and come up with something really stable?”
Kroto remembered he had built with his children a paper star dome that consisted of both pentagons and hexagons. He wanted to call his wife in England to have her find the construction.
“But it was getting late, and it seemed highly improbable that he had done this,” Curl said.
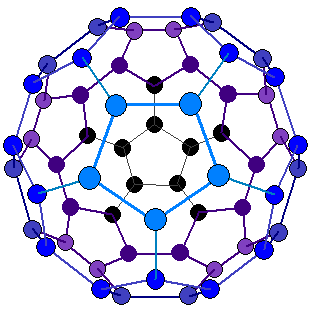
Instead, that night, Smalley fiddled with paper, scissors and scotch tape, creating a paper sphere made up of 20 hexagons and 12 pentagons with 60 corners. It fit all the parameters for a stable form of carbon with 60 atoms.
The structure resembled the geodesic domes that American architect Buckminster Fuller designed for the 1967 Montreal World Exhibition. They decided to name the structure buckminsterfullerene in his honor. They called the spheres Buckyballs for short because they resembled soccer balls.
The trio was excited about what they came up with, but it was only a theory. They had no proof other than the high number of C60 molecules they were seeing in their experiments.
“That didn’t deter us,” Curl said.
Read the rest, it’s worth your time. Smalley passed away in 2005; Curl is now an emeritus professor. They didn’t have to win the Nobel for this research – there was another team that had made a similar discovery. Buckyballs themselves were never of much practical use, but the discovery led to the field of nanotechnology and the creation of nanotubes, among other things. It’s fair to say we live in a different world today because of Robert Curl and Richard Smalley.

Don’t forget about Sir Harry Kroto, the third recipient of the Nobel for the discovery of C-60, who just passed away last month. It was his ideas about interstellar chemistry that led the trio to the discovery.
Very worth watching is this moving tribute by one of his British friends and colleagues, chemist Sir Martyn Poliakoff: https://www.youtube.com/watch?v=n0ADOfaxWYc